Every machine requires particular components and fuel in order to operate, according to the Cellular Respiration Equation. Similarly, in order to operate, biological machines need well-engineered components and a sufficient energy supply. Adenosine triphosphate (ATP) is perhaps the second most significant molecule (after DNA). ATP is essentially the cell’s primary energy currency.
What is Cellular Respiration?
Living creatures, such as plants, animals, and microorganisms, create their own energy using a process known as cellular respiration. Depending on the type of precursor used to create ATP, different species may be divided into two groups:
- A) Aerobic organisms are those that use oxygen in their daily lives.
- B) And – those who do not are described as “anaerobic“.
Why is Cellular Respiration Important?
Cellular respiration (aerobic or anaerobic) produces enough ATP for sustaining organisms, as previously stated. ATP may then be used to power a variety of intra-cellular physiological operations, including the movement of molecules across cell membranes and the synthesis of biological molecules.
Aerobic Respiration
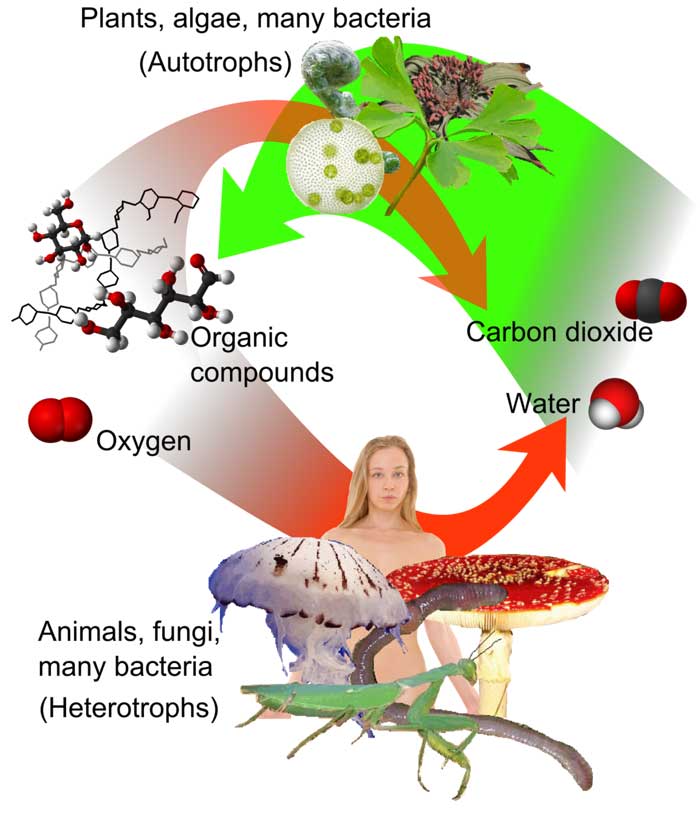
Aerobic respiration is the term for cellular metabolism that requires oxygen. Among all types of cellular respiration, it is the most efficient. Photosynthesis supplies plants the precursor chemicals, whereas the food they consume (i.e., plants and animals) provides them with this form of respiration. The example of this is plants and animals.
Photosynthesis Equation
The equation for photosynthesis of plant cells is:
6CO2 + 6 H2O + Sunlight → C6H12O6 + 6O2
( 6 Carbon Dioxide + 6 Water + Sunlight → Glucose + 6 Oxygen )
Cellular Respiration Equation
It’s worth noting that cellular respiration is a collection of metabolic processes, not a single process. As a result, each pathway’s locations in the cell are different. The overall chemical reaction of the cellular respiration equation is represented as follows:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + 38ATP
( Glucose + 6 Oxygen → 6 Carbon Dioxide + 6 Water + ATP )
* Value is not constant for all aerobic organisms. May range from 34 to 38 net ATP.
By putting the three following procedures into a single procedure, the above cellular respiration formula is created. The procedures discussed below are described in detail.
Stages of Cellular Respiration
1. Glycolysis

Glycolysis is the first metabolic pathway that occurs during cellular respiration. Glycolysis is the breakdown of one glucose (sugar) molecule into two pyruvate molecules, and it is derived from the Greek word “glyk” which means “sweet” and “lysis.”
- Glycolysis occurs in the cytosol, as shown in the illustration above.
- The simplified equation for glycolysis is: ten enzyme-catalyzed reactions
C6H12O6 + 2 NAD+ + 2 ADP + 2 P → 2 pyruvic acid,
(CH3(C=O)COOH + 2 ATP + 2 NADH + 2 H+
- Glycolysis creates two ATP molecules, but the whole procedure generates four, according to the aforementioned equation. Nevertheless, during the preparatory stage, two molecules are used up, resulting in a net gain of just two ATP molecules.
- There is a so-called “link reaction” that occurs after glycolysis. The Pyruvate dehydrogenase complex (PDC) catalyzes the oxidation of pyruvate to CO2.
- One molecule of NADH (nicotinamide adenine dinucleotide) and one molecule of carbon dioxide are produced when the pyruvate from glycolysis is oxidized (converted).
2. Krebs Cycle
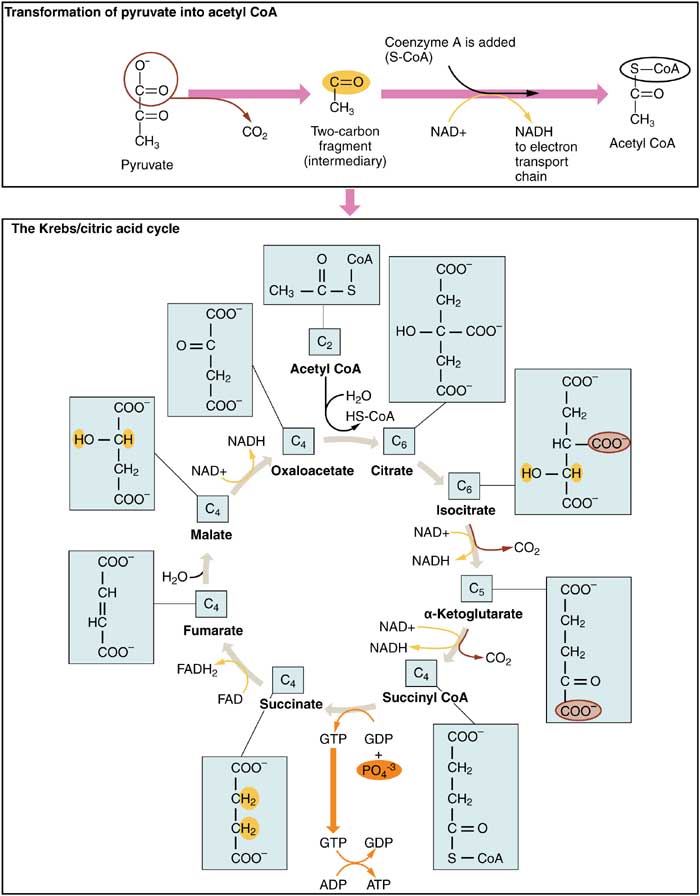
The Krebs cycle (named after Hans Adolf Krebs) is an eight-step procedure that uses 18 different enzymes and is also known as the Tricarboxylic Acid (TCA) cycle or simply the Citric Acid cycle.
- A succession of oxidation-reduction processes leads to the oxidation of the acetyl group to two carbon dioxide molecules in the Krebs cycle, which takes place in the mitochondrion’s matrix.
- A net of 3 NADH, 1 FADH2, and GTP (guanosine triphosphate) occurs throughout one cycle.
- As a result, a total of 6 NADH, 2 FADH2, and 2 ATP molecules are produced from one glucose molecule (which formed 2 pyruvate).
- It’s worth noting that carbon sources are used to produce high-energy electrons in the Krebs cycle. Also note that the process does not use oxygen as a precursor molecule and does not generate a lot of ATP.
- Instead, it forms NADH and FADH2 by extracting electrons from acetyl coA.
3. Electron Transport Chain and Oxidative Phosphorylation
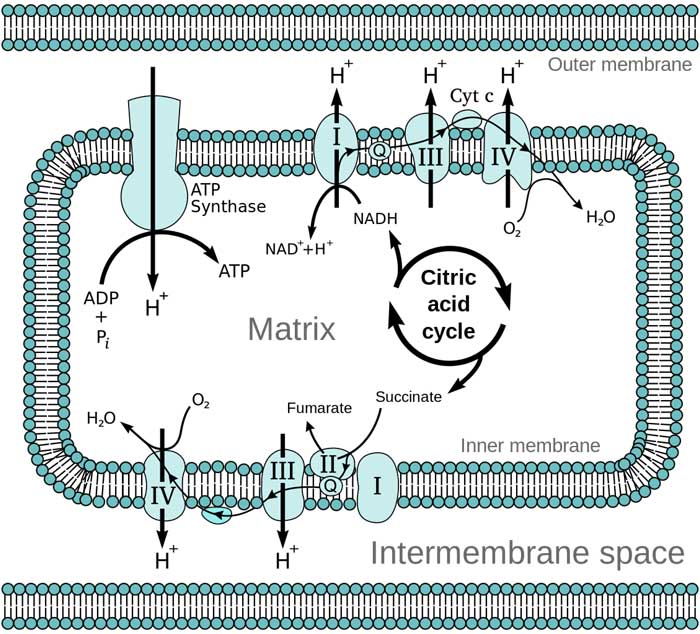
The final route in cellular respiration (PDF) is made up of the electron transport chain and oxidative phosphorylation, both of which occur in the inner membrane of the mitochondrion.
- By oxidizing the NADH from the Krebs cycle, this former, which is a component of the latter, creates the chemiosmotic gradient (proton gradient) across the inner membrane of mitochondrion.
- Electrons are transferred from one complex to the next in ETC, where they consume oxygen and generate water. During cellular respiration, such responses generate the vast bulk of ATP.
- Up to 34 ATP per molecule of glucose is produced by ETC, which also produces water, NAD, and FAD (which are both recycled back to glycolysis).
From a single glucose molecule, aerobic cellular respiration can produce up to 38 ATP in total. Certain species, on the other hand, have a distinct precursor molecule and can only generate 34 to 36.
What is the role of Oxygen in the process?
Oxygen is an essential molecule in cellular respiration. But where does it exactly fit in the picture? Basically, oxygen can be found at the end of the ETC (during aerobic respiration) where it accepts electrons while picking up protons in order to produce water molecules.
- As a result, oxygen is known as the “final electron acceptor.” The electron transport chain will stop when oxygen levels are reduced since electrons will be dispersed easily.
- Of course, due to the lack of ATP, certain cellular activities will be halted.
Fermentation
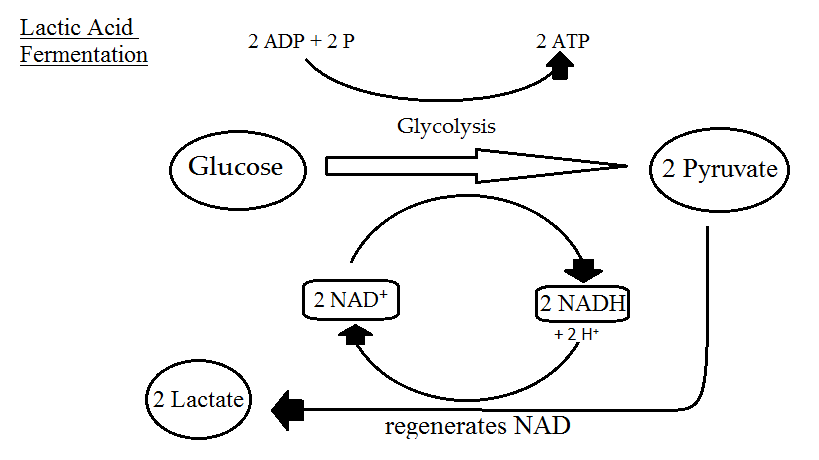
alternatively, the cell may perform a process known as fermentation and use a different pathway at the end of glycolysis when there is insufficient oxygen. Instead of oxidative phosphorylation, it employs substrate-level phosphorylation, which does not need oxygen in the process (not to be confused with anaerobic respiration).
Lactic acid fermentation is the name for this process in animal cells. Except for the fact that it generates lactic acid, it’s practically identical to aerobic respiration. In the equation, it is possible to simplify it as follows:
C6H12O6 → 2 CH3CH(OH)COOH + 2 CO2 + 2 ATP
Microorganisms, on the other hand, such as yeast, produce ethanol and carbon dioxide. The ethanol or alcohol fermentation is the term used for this process.
C6H12O6 → 2 C2H5OH + 2 CO2 + 2 ATP
Just two ATP are generated from a glucose molecule in both sorts of fermentation processes.
Anaerobic Respiration

This process is similar to a typical cellular reaction (same glycolytic and Krebs cycle pathways) in that it uses oxygen as the final electron acceptor, but it differs due to the fact that bacteria and archaea use it. Instead, sulfates and nitrates are used by these organisms.
- It’s essential to understand that anaerobic fermentation is just an alternative to glycolysis, while the former extends glycolysis and generates energy when the organism dies in the presence of oxygen.
- Aerobic respiration takes place in the cytosol, not the mitochondria, as does aerobic respiration.
- Just 2 ATP are produced per glucose molecule during the anaerobic respiration process.
Living organisms should create ATP, which gives them energy and allows them to carry out all of their metabolic and activities. In addition, if a single molecule or enzyme is missing from the cellular respiration equation, it will be unable to proceed. If they aren’t, metabolic chaos ensues.
